Department Atomic-Scale Dynamics in Light-Energy Conversion
Gallery
11.1.2023: Hanna’s first first-author paper!
Congratulations Hanna! Our paper about the reversible and irreversible cation intercalation in NiFeOx oxygen evolution catalysts in alkaline media was published in The Journal of Physical Chemistry Letters. Together with our colleagues from the Leiden Institute of Chemistry at the Leiden university, The Netherlands, and from HZB, we studied the intercalation process by measuring the soft X-ray absorption spectra at the nickel L-edge as well as at the sodium and oxygen K-edges of this layered Ni0.8Fe0.2Ox electrocatalyst during the oxygen evolution reaction using NaOH as electrolyte. The spectra reveal a (reversible) increase in the sodium intensity at the intercalation potential, which also coincides with an increase in the nickel oxidation state. However, the reversible increase in intercalated Na+ ions did not lead to an increase in the water content of the catalyst layer, which suggests that the intercalation of Na+ has little effect on the catalyst morphology. Our work also supports previous electrochemical studies that Na ions from the electrolyte are being intercalated into the γ-phase of Ni-based electrocatalysts for water oxidation. For more information about this fascinating story, please click here.

A) Sketch and b) photograph of the dip-and-pull setup with the yellow iron hexacyanide solution in a beaker sitting in the vacuum chamber under ambient pressure conditions. The three electrodes are first c) dipped into the electrolyte solution and then d) pulled up, with measurements taken while slowly moving between positions d) and e). The liquid meniscus is clearly shown in the photograph of f). The dashed circle in b) highlights some small hydrogen bubbles at the counter electrode. © J. Chem. Phys. 157, 244701 (2022)
22.12.2022: Our spectroelectrochemistry paper about operando measurements on iron hexacyanide using the dip-and-pull technique has been published in the Journal of Chemical Physics.
What a nice Christmas present! Our ‘multi-synchrotron’ work between MAXIV, Lund, and BESSY II, Berlin, on X-ray photoelectron spectroscopy of the redox reaction of iron hexacyanide has been published in JCP as part of the Special Topic on ‘In situ and Operando characterization’. While our colleagues around Robert Temperton from the HiPPIE beamline at MAXIV performed ambient-pressure XPS measurements using the so-called dip-and-pull technique to study the conversion of [FeIII(CN)6]3− in water into [FeII(CN)6]4− and following the (reversible) iron 2p signal, Robert and Wilson at HZB measured the reference iron 2p spectra with the liquid-jet technique for both redox states with the SOL³PES setup at BESSY II. The dip-and-pull technique is a fairly new technique in which a thin liquid film is prepared by pulling an electrode out of a degassed solution beaker in a ‘vacuum’ chamber equilibrated by the vapor pressure of the electrolyte solution (in case of water, ~10 mbar at 10°C). Our study demonstrates that the liquid film on the electrode is electrochemically active such that the relative potential difference between the working electrode and the solution can be controlled to a large extent. For more information about this technically demanding experiment, please click here.
29.11.2022 Visiting LIDUX Laser Lab
Robert has visited Iain Wilkinson in his new state-of-the-art ‘Liquid and Interfacial Dynamics with Ultrafast X-rays facility’, LIDUX, at the Wannsee campus of HZB. Liquid jet and flatjet experiments with this impressive, powerful 500 W laser system will be available by the end of 2023. It will then be possible to observe ultra-short femtosecond processes after UV/Vis excitation by probing them with few-femtosecond soft X-ray pulses, capable of kicking out electrons from deeper lying core-orbitals that offer much cleaner spectral fingerprints compared to electrons ejected from the valence band. This laser offers the excellent time resolution of an X-ray free-electron laser, but does not generate any unwanted space charges in the samples due to lower pulse intensity and a much higher repetition rate of 53 kHz. Check out more here.
17.11.2022: Garlef Wartner is now Dr. Garlef Wartner!
Congratulations Garlef! With many, many spectroscopy-related questions from the PhD committee, consisting of the reviewers Simone Raoux (HZB/Humboldt University), Olle Björneholm (Uppsala University), and Robert, as well as the Humboldt University professors Oliver Benson, Emil List-Kratochvil, and Jan Lüning, Garlef delivered a brilliant performance in his defense. Overall, his PhD was graded with magna cum laude. Garlef will continue his work on electrochemical water splitting-cells in the group of Marc Tesch from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr, but will stay in Adlershof and work in collaboration with the Fritz Haber Institute Berlin and HZB. So, it is not time to say goodbye yet.
4.11.2022: Beamtime at MAXIV
Robert joined Kevin Lovelock and his team from Reading University for a four-day beamtime at MAXIV in Lund, Sweden. We studied the resonant Auger spectra of zinc salts in different solvents and were particularly interested in the participator Auger fingerprints of the anions and looked for trends in the (valence) binding energies upon changing the solvent. This work began in an earlier beamtime by Kevin Lovelock at BESSY II with the SOL³PES and is now being continued at the HiPPIE endstation at MAXIV, which is managed by Robert Temperton. After careful data analysis by the PhD students Lewis and Francis, the data from both synchrotron beamtimes will (hopefully) find their way into a publication.
26.10.2022 Beamtime at UE52-SGM
Our colleagues and users from Fritz Haber Institute Berlin, the University of Chemistry and Technology, Prague, and from the University of South Bohemia, Czech Republic, were allocated a beamtime at the polarization-rotatable beamline UE52-SGM with our SOL³PES station. They used seven night shifts to measure the anisotropy in the photoelectron angular distribution (PAD) of solutes in aqueous solutions using a cylindrical liquid microjet and a flatjet. PADs reveal information on the orbital character and symmetry from which an electron is ejected. Furthermore, PADs have great relevance for depth-profile measurements, which are often applied for identifying the species existing at the vacuum-solution interface. While in the past PAD measurements have been performed on liquid microjets, PAD measurements from a flatjet have never been performed before. There is an expectation that a flat surface will not interfere with the PAD compared to a cylindrical jet. However, the measurements rely crucially on the jet stability. Fortunately, Bruno and Dominik from FHI Berlin are experts in handling flatjets and the first (raw) data looks very promising to derive the ‘true’ anisotropy from the aqueous samples.
7.9.2022: Martin Schellenberger successfully defended his PhD. Congratulations!
Well done, Martin! He showed a strong performance in the defense and could answer all questions with ease. The doctoral committee members, consisting of the three reviewers, Prof. Philipp Adelhelm from Humboldt university Berlin, Prof. John Hemminger from University of California, Irvine, and Robert, as well as the Humboldt university professors Kallol Ray, and Nicolas Pinna, were impressed and graded the defense (talk + oral examination) with summa cum laude (the highest possible grade). The overall grade for his PhD work is magna cum laude. Martin graduated in three years and will continue his work as postdoc in the group of Dr. Sebastian Risse from HZB. So it is only half goodbye!
18.8.2022 Our article about intermolecular Coulombic decay in liquid ammonia has been published in Structural Dynamics.
Our colleagues from FHI (Winter group) and from Prague (Slavíček group and Hanns Christian Schewe from Jungwirth group) together with Robert measured Auger spectra of liquid ammonia for the first time. The intermolecular Coulombic decay (ICD) fingerprints are hidden under a shoulder on the high-energy side of the normal nitrogen Auger peak overlaid by shake-up satellites. In ICD, an initially core or inner-valence ionized atom or molecule electronically decays, and the released energy ionizes a neighboring entity via Coulombic electron interactions. Thanks to the theorists from Prague, we were able to deconvolute the messy Auger-peak structure, and our calculations support that ICD does indeed contribute. Liquid ammonia is a hydrogen-bonded system, and the electronic processes can couple to nuclear dynamics following core-level ionization. Unlike in liquid water, we found no significant proton-transfer dynamics occurring between the hydrogen-bonded units within the respective N 1s core-hole lifetime. You can read more about this fascinating basic-research study here.
27.7.2022 Gordon Research Conference in Holderness
After a three-year hiatus due to the Corona pandemic, Robert attended the Gordon Research Conference of Water and Aqueous Solutions in Holderness, New Hampshire, USA. He presented recent liquid microjet data from sulfuric acid in water. From the valence and core-level spectra we can derive the dissociation constant as a function of probing depth, temperature and concentration. These dissociation constants are important input parameters for various climate (change) models, since aqueous aerosols of sulfate and bisulfate ions play a crucial role in cloud formation.
20.6.2022 HZB retreat in Schmöckwitz
Our annual 2-day retreat for all group leaders at the HZB took place in beautiful Schmöckwitz, located at the very east of Berlin on the border to Brandenburg. A tightly packed schedule with talks, presentations and group discussions on the strategies and future plans of BESSY II and III awaited Renske and Robert.
1.5.2022: We joined PS-ADLU!
We joined Renske van der Veen's department of Atomic-Scale Dynamics in Light-Energy Conversion (PS-ADLU) at HZB!
6.4.2022: Hanna got beamtime at BESSY II
Hanna was granted a one-week beamtime at the U49/2-PGM-1 beamline, where she measured iron-nickel (oxy)hydroxides under operando conditions using the Lixedrom endstation from Ronny Golnak. The goal was to observe in-situ the intercalation process of sodium ions in Ni0.8Fe0.2Ox during the oxygen evolution reaction and its impact on the hydration of the electrocatalyst. For these measurements, Hanna used a specialized electrochemical cell, designed by Ronny Golnak and Marc Tesch. Thanks to an updated and improved beamline software, a much faster scan rate (the so-called fly mode or continuous mode) could be used to measure the absorption spectra in a few minutes, which previously took almost an hour.
18.3.2022: First PES measurements of Naphthalene anion radicals in liquid THF
The groups of Pavel Jungwirth (IOCB Prague), Stephen Bradforth (USC Los Angeles), and Bernd Winter (FHI Berlin) as well as Robert measured for the first time photoelectron spectra of naphthalene anion radicals in cooled Tetrahydrofuran solutions in presence of (dissolved) sodium metal. This beamtime is part of our cryo-liquidjet project, where we want to investigate the electronic structure and spectroscopic fingerprints of aromatic intermediates during the Birch reaction. Since any water ‘kills’ the reaction, much effort was put into purifying THF prior to the measurements at the beamline.
4.3.2022: Robert Temperton from MAXIV visited us and measured the participator Auger spectra of high-spin transition metal complexes
Robert Temperton got three night shifts for his ligand-field investigations of iron and manganese salt complexes in different solvents at the SOL³PES. From the metal L-edge absorption and resonant valence band photoelectron spectra, Robert is able to identify the energetic positions and the changes of the participator Auger lines, which are directly related to the metal 3d orbitals hybridized to the ligand orbitals. It was a classic liquid-microjet beamtime that did really well.
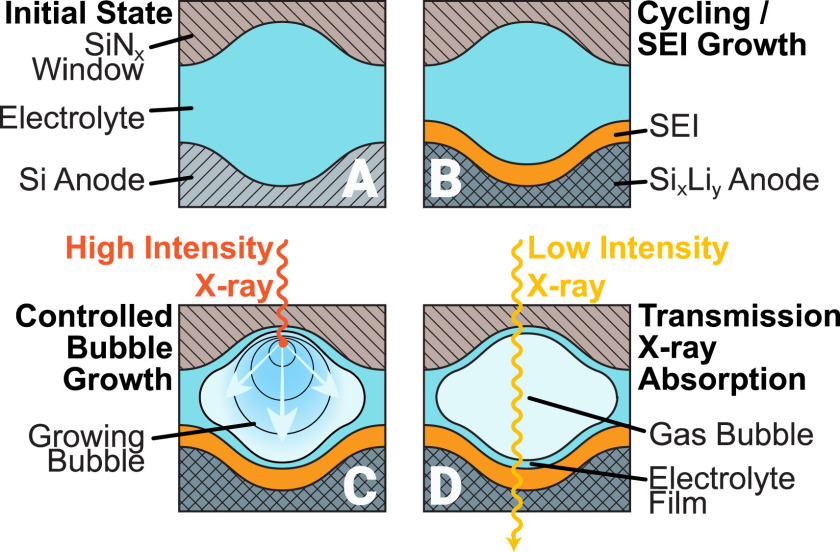
To study the Solid-Electrolyte-Interphase with soft X-rays in transmission mode, we used a novel ‘bubble’ approach to remove most of the electrolyte in the electrochemical cell, leaving only a thin electrolyte film of electrolyte on the battery anode. © Schellenberger et al., materialstoday Advances 2022, 14, 10021, DOI: 10.1016/j.mtadv.2022.100215
12.2.2022: Our article about the quasi in situ investigations of the solid-electrolyte interphase on silicon-anode based lithium-ion batteries has been published in the journal Materials Today Advances
Congratulations Martin! This paper is the well-deserved success of all the hard work during the last three years in this project. It summarizes three major accomplishments: 1) We introduced a novel approach for true transmission spectroscopy on liquids and thin-film battery electrode materials in the soft X-ray regime under in-situ conditions. Our unique method puts a twist to conventional SiNx window-based liquid cells by utilizing a deliberately induced gas-bubble to form a soft X-ray transparent thin electrolyte layer. With this new approach we investigated the solid-electrolyte interphase composition on silicon thin-film anodes. 2) Based on spectral fingerprints at the oxygen K-edge, we identified acetates (based on Li acetate and Li trifluoroacetate), Li ethylene carbonates (based on LEMC and LEDC), acetonates (based on Li acetylacetonate), and LiOH as the solid species which are most likely to be present in the solid-electrolyte interphase on silicon anodes in thin-film lithium-ion batteries. 3) At the fluorine K-edge we identified LiF to be the main fluorine-containing species in the SEI. Besides this, we suspect that the acetate species may be fluorinated and hence can be correlated to the observed trifluoroacetic acid fingerprint. Furthermore, even though reported in the literature, from our oxygen K-edge and silicon L-edge spectra we could not find clear evidence of lithium silicates or lithium phosphates. For more information please click here.
3.2.2022: Our article about photoelectron angular distributions as sensitive probes of surfactant layer structures at the liquid-vapor interface has been published in PCCP
Our friends and colleagues from the Fritz-Haber institute, the Bluhm group and the Winter group, and Robert from HZB measured last year with our SOL³PES setup at the UE52-SGM beamline at BESSY II the photoelectron angular distributions (PADs) of octanoic acid in water. This model surfactant is an ideal candidate to test the sensitivity of PADs for probing depth information relative to the liquid-vapor surface for carboxylate (COO-) and carboxylic acid (COOH) groups, both of which are present aqueous solution depending on the pH value. We found that the COO- groups are deeper immersed than the COOH groups. This was also verified by our theory-co-authors Jakob Filser and Harald Oberhofer from the Technical University in Munich, who performed classical molecular dynamics simulations. More about this fascinating topic can be found here.
22.12.2021: Our article about the photoelectron spectroscopy of benzene in the liquid phase and dissolved in liquid ammonia has been published in the Journal of Physical Chemistry B as part of the virtual special issue “Dor Ben-Amotz Festschrift”.
Continuing the successful cryo-liquid spectroscopy story at our SOL³PES setup, Pavel Jungwirth and his team from Prague together with Bernd Winters group from the FHI Berlin as well as Wilson and Robert from HZB have recorded the valence band spectra of pure liquid benzene as well as benzene dissolved in liquid ammonia for the first time. Using an updated experimental kit from Christian Schewe, we were able to bias the cryo-jet, which made an absolute binding energy calibration of our spectra possible. We observed large solvent-induced shifts in the valence electron binding energies of benzene, which can be explained by the Born-Haber solvation model. We could also explain why these shifts are practically the same in liquid ammonia and in benzene, although both liquids have very different static dielectric constants. Have a look of this ‘cool’ story.
18.12.2021 Our first users from outside the European Union after two years.
It was about time that we could welcome again users from outside the European Union since the Corona pandemic started. PhD student Anne McGrogan from Gosia Swadźba-Kwaśny’s group at Queen's University Belfast, Northern Ireland and PhD student Lewis Parker from Kevin Lovelock’s group at the University of Reading, England, had been granted a one-week beamtime at our SOL³PES setup. They traveled without their PI’s as the Corona restrictions currently allow only two external users at the beamline. Together with Robert, Anne and Lewis investigated various boron-containing ionic liquids in methanol, and in acetonitrile with the liquid jet technique. Due to their decomposing nature, the solutions had to be kept under nitrogen gas at all time. Thanks to Ronny Golnak, we could use a mobile nitrogen glove box, from where our solutions were loaded into the HPLC system.
3.12.2020: Dennis Hein is now Dr. Dennis Hein.
Congratulations Dennis! He gave a marvelous defense talk and the subsequent oral examination was flawless, which was graded with summa cum laude (the highest possible grade). The doctoral committee, consisting of the three reviewers, Prof. Simone Raoux from HU and HZB, Prof. Olle Björneholm from Uppsala university, and Robert, as well as the HZB boss Prof. Jan Lüning, and the chairman Prof. Christoph Koch from the Humboldt university, were truly amazed by Dennis’ performance. The overall grade for his Phd work is magna cum laude. Dennis graduated in less than 3.5 years and will now take on new challenges in the industry. We will miss you and wish you all the best in your future endeavors.
22.11.2021: Beamtime at PETRA III with EASI setup
We were granted a few shifts at Bernd Winter’s EASI setup, currently attached to the P04 beamline at the PETRA III synchrotron in Hamburg. We investigated nickel oxide- and iron-nickel-oxide catalysts for the oxygen evolution reaction with our micro-electrochemical cell under open-circuit and applied voltage conditions. The catalysts were deposited as mesoporous layers on top of an ionomer membrane that is (semi-)transparent for water and ions, but withstand the pressure difference of 1 bar between the electrolyte and the vacuum. We measured absorption and resonant photoelectron spectra from the catalyst-water interface and performed at the same time cyclovoltammetric measurements. Hanna is happy about her first operando PES data.
2.10.2021: Pavel Jungwirth and his team were granted a one-week beamtime at our SOL³PES setup.
After studying liquid ammonia, liquid benzene and liquid benzene in liquid ammonia, as well as alkali metals in liquid ammonia, we went for the ultimate experiment: We tried to spectroscopically follow the Birch reaction, i.e., the reduction of the aromatic benzene ring in liquid ammonia in the presence of lithium to form the benzene anion radical. The experiment is tough – the solubility of benzene at low temperatures and the cryo-jet stability at higher temperatures exclude each other. The spectra taken are too noisy, but we learnt a lot and will improve in the next beamtime.
16.9.2021: Bernd Winter and his group were granted a one-week beamtime at our SOL³PES setup.
Bernd and his team measured with an upgraded flat-jet setup the photoelectron spectra of aqueous solutions and the binding energy-shifts when applying a bias. The flat-jet is created by two liquid-microjets colliding under an angle and produces a leaf-like flat surface ideal for photoelectron spectroscopy investigations. By using different (conductive) solutions for the two liquid-microjets fundamental questions regarding the liquid-liquid interface and the surface potential in the flat-jet can be studied.
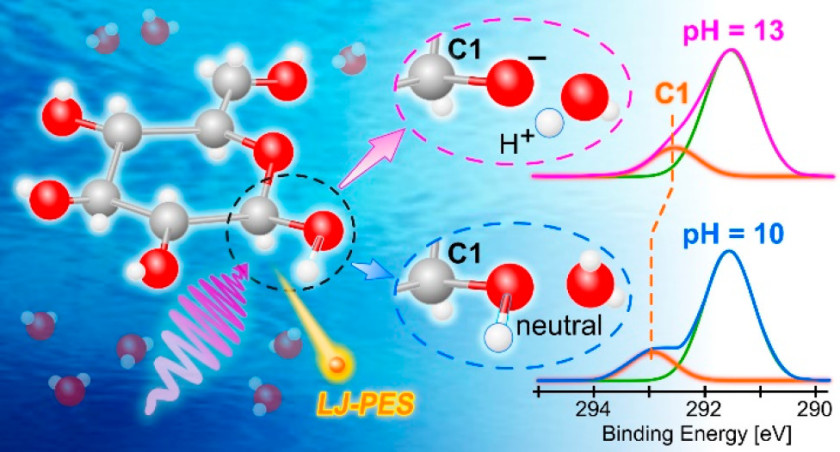
30.07.2021: Our article ‘Following in Emil Fischer’s footsteps: a site-selective probe of glucose acid–base chemistry’ has been published in the Journal of Physical Chemistry as part of the virtual special issue “Daniel Neumark Festschrift”.
Bernd Winter and co-workers performed liquid microjet measurements with our SOL³PES station in order to determine the first acid dissociation constant of glucose in water and at the same time to identify the spectroscopic signature of the respective deprotonation site. We measured the photoelectron signal of the carbon-1s peak as a function of pH – in a sense it can be considered a photoemission spectroscopy titration. This approach will now be used to investigate the acid-base chemistry and structure-function relationship of other polyprotic acids. To find out more about this intriguing work, please click here.
28.7.2021 Our article about spectroscopic evidences for a gold-coloured metallic water solution has been published in Nature!
What a beautiful golden color! Led by Pavel Jungwirth, a strong collaboration of 15 scientists from IOCB Prague, Fritz-Haber institute Berlin, USC Los Angeles, Berkeley university, Kyoto university, and Helmholtz-Zentrum Berlin, had produced for the first time a metallic water solution. This proves that also water, an almost perfect insulator, can be turned into a metal-behaving conductor. Usually, extreme pressure conditions such as one can find only in the cores of larger planets are necessary to squeeze the outer electrons of any material hard enough to form a conduction band and to develop metallic properties. Lacking the possibility to achieve the necessary pressures on Earth, we came up with a different idea to produce metallic water – by condensing water vapor on top of alkali metal drops, which releases their outer electrons very quickly into the thin water layer. The released electrons in the water behave like free electrons in a conduction band. We investigated the metallic water solution by soft X-ray photoelectron spectroscopy with our SOL³PES setup at BESSY II and by optical reflection spectroscopy at the IOCB in Prague, where we unambiguously found the spectral fingerprints, the plasmon binding energy at 2.7 eV and the conduction band, including a sharp Fermi-edge. More about this fascinating study can be found here, and here. Philip E. Mason also made a nice Youtube video from the experiment and the golden droplet formation. Click here for the video.
7.7.2021 Our article about the spin propensity in resonant photoemission of transition metal complexes has been published in the new Journal Physical Review Research.
Together with our collaborator Robert Temperton from Lund/Max IV and his coworkers we digged deep into the resonance behavior of iron hexa cyanides in the 2+ (closed-shell) and in the 3+ (open shell) oxidation state. By sweeping the photon energy between 706 eV and 716 eV, we could resonantly excite an electron from the iron 2p3/2 orbital into the 3d valence band, and follow the spin-coupling processes. For example, the autoionization of [FeIII(CN)6]3− shows a dramatic fourfold singlet versus triplet final-state enhancement in the resonant photoelectron spectrum. Our study proves that the ability of resonant photoelectron spectroscopy to observe orbital degeneracy and spin-propensity sets out a platform for using RPES mapping to resolve otherwise inaccessible spin configurations, which has fundamental importance in the understanding of the electronic and spin properties of open-shell or high-spin materials. More details can be found here.
21.6.2021 CatLab officially started!
The Catalysis Laboratory, in short CatLab, has started! It bundles the competences of the Helmholtz-Zentrum Berlin and the two Max Planck Institutes, Fritz-Haber institute Berlin and the institute for Chemical Energy Conversion in Mühlheim to boost the development of new thin-film catalyst-materials for the production of green hydrogen on a macroscopic scale. It is funded by the Federal Ministry of Education and Research with about 51 million euros. Hanna Trzesniowski will be part of CatLab through a Phd-funded position. More news about the CatLab project can be found here.
15.6.2021 Hanna Trzesniowski starts her Phd in our group!
Being a research assistant the last 12 months, Hanna will now continue her work on meso-porous thin-film electrocatalysts as Phd student in our group. We wish her permanent success!
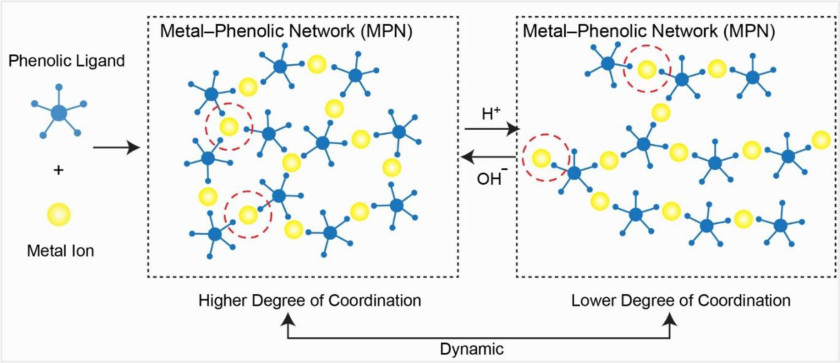
Our TOC figure sketches the mechanism behind the impressive buffering capacity of MPNs. ©Chem. Mater. 2021, 33, 7, 2557–2566, DOI: 10.1021/acs.chemmater.1c00015
22.3.2021 Our article about the Metal-Phenolic Networks as Tunable Buffering Systems have been published in the Journal Chemistry of Materials.
Our collaborator Sebastian Beyer from the Chinese University of Hong Kong, our colleagues from the University of Melbourne, and Robert could show analytically and spectroscopically that metal-phenolic networks (in short MPNs) exhibit twofold higher buffering capacities than polyelectrolyte complexes and fourfold more than commercial buffer solutions. The buffering effects are driven by pH-responsive, multivalent metal-phenolic coordination. We could demonstrate that the MPN buffering effects are retained after deposition onto solid supports, which allowed for a stabilization of aqueous environmental pH for more than one week. For more details please click here.
1.1.2021 EE-NOGP is now CE-NOGP!
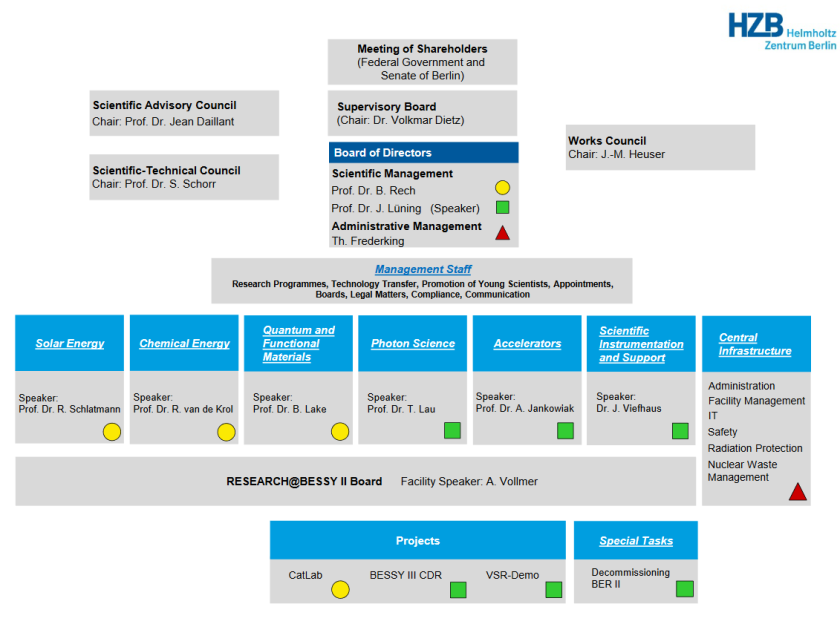
Since January 1st 2021, HZB has a new organizational structure. There are now six scientific divisions plus the central infrastructure division. Our research group CE-NOGP, which stands for Chemical Energy Division – Young Investigator Research Group Operando Interfacial Photochemistry (in German Chemischer-Energie-Bereich – Nachwuchsgruppe Operando Grenzflächen-Photochemie), is located in the Chemical Energy Division with the speaker Roel van de Krol. Our responsible scientific manager from the board of directors is Bernd Rech. The new structuring became necessary during the POF-IV application process and it also considers the differences between solar energy and solar fuels/battery research, which were before 2021 part of the renewable energy division.
15.10.2020 Dennis’ first first-author paper – well done!
We investigated the Reversible Water-Induced Phase Changes of Cobalt Oxide Nanoparticles and published the results in ACS Nano! Together with our former postdoc Arno Bergmann and his new group leader, Beatriz Roldan Cuenya, as well as a former PhD student from Beatriz’ group, Miguel Bernal, we measured the electronic-structure changes of highly catalytically active core-shell cobalt-oxide nanoparticles under high vacuum and near-ambient pressure conditions. We found out that in the presence of water the cobalt oxide changes its phase from the tetrahedrally coordinated form to octahedral coordinated Co2+ and Co3+ – and this oxidation change is reversible, once the water is removed. This is in contrast to previous findings, which suggested an irreversible phase change of tetrahedrally coordinated Co2+ after the oxygen evolution reaction conditioning. Our results demonstrate the appearance of water-induced structural changes different from voltage-induced changes and help us to understand the atomic scale interaction of CoOx nanoparticles with water in electrochemical processes. For more information, please check this link.
30.9.2020 Beamtime at DESY Hamburg
Hanna, Dennis, Garlef and Wilson had been granted beamtime at the PETRA III synchrotron radiation facility in Hamburg, using Bernd Winters new EASI setup. Dennis, Garlef and Hanna measured successfully during the dayshift resonant photoelectron spectra of iron-nickel oxide catalysts on top of a graphene-bilayer inside a micro-electrochemical cell under operando conditions. Together with Florian Trinter from Bernds group, Wilson performed during the nightshift liquid-jet studies on the precursor solutions, which we used for electro-depositing our iron-nickel catalyst onto the graphene bilayer. These measurements went smooth and flawless. It was a good beamtime!
8.9.2020 Our article about the electronic structure of the aqueous permanganate ion has been published in PCCP
Karen Mudryk from Iain Wilkinson’s group at HZB performed extensive liquid-jet measurements at the SOL³PES station on this very important textbook anion – permanganate, MnO4-, in water – revealing its most sacred and secret electronic-structure information. Permanganate solutions are very corrosive and highly oxidizing, making it difficult to investigate the solutions at high concentrations. With the support of Iain, Bernd Winter, and Robert this paper became a very dense and informative article, answering fully all aspects about the binding energies, Auger-electron spectra, bulk/surface propensities, counter ion dependence, concentration effects, and redox properties of MnO4-. For more information check this link.
And there is more to come: Karen also performed measurements on the manganate ion, MnO42- in water, which is the reduced form of permanganate. The results will be published soon.
2.7.2020 Our article about the valence photoelectron angular distribution of water has been published in the journal of physical chemistry letters.
It took 6 years from the first angle-resolved measurements at BESSY II in July 2014 to the publication in JPCL in July 2020. Five beamtime campaigns and numerous versions of the manuscripts written led to a really nice and important study about the anisotropy parameter beta for the three valence orbitals of water. The anisotropy parameter indicates how much the photoelectron angular distribution differ from a pure isotropic distribution, when water is hit by linear polarized light. We found that the outgoing photoelectrons from the three valence orbitals of liquid water have a ~20% decrease in the anisotropy between 250 and 750 eV kinetic energy compared to the gas phase. With the help of our theory collaborators from the US, Anna Krylov and Samer Gozem, we could show that this reduction in the anisotropy is primarily due to scattering rather than rehybridization due to solvation. This is in line with a previous angular distribution study of water’s 1s orbital, which we published in 2013. Thanks to Uwe Hergenhahn and Evgeny Lugovoy from Bernd Abels group we also have angular resolved data for 38 eV photon energy, measured with a high-harmonic generation laser source at IOM Leipzig, which helped to refine our model for the low-kinetic energy regime. For more details of this very fascinating basic-research study of liquid water please check this link.
15.6.2020 Welcome Hanna Trzesniowski!
Research assistant Hanna Trzesniowski from Graz, Austria, joined our team. A very warm welcome!
5.6.2020 Our article ‘Photoelectron spectra of alkali metal–ammonia microjets: From blue electrolyte to bronze metal’ has been published in Science and got also the cover story.
Together with our colleagues from IOCB Prague, Charles University Prague, USC Los Angeles, FHI Berlin, and University of Kyoto, we investigated the gradual formation of a conduction band in an electrolyte by using liquid microjet photoelectron spectroscopy at our SOL³PES setup. We dissolved lithium and sodium metal in cryogenic, liquid ammonia, leading to solvated electrons, which are very stable – even at high concentrations in this solution. With increasing metal concentration, the solution color changes from blue to golden, indicating a transition to a metallic compound. The conductivity of this metallic-behaving golden solution is even higher than the conductivity of the lithium or sodium metal, which has been dissolved in this polar, non-conducting solvent. By tracking the spectral fingerprint of the solvated electron with increasing metal concentration we observed that the narrow peak widens into a band with a sharp Fermi edge in the spectrum, which is characteristic of metals, accompanied as well by signals associated with collective excitations (plasmons) – characteristic of free metallic electrons. This study was a tour de force and has been published in Science and also got the cover story. Well done everyone.
Phil Mason, one of the co-authors, made two fantastic Youtube videos of this endeavor, click here and here. They nicely summarize what we have measured and why it is so noteworthy that it ended on the cover of Science.
9.4.2020 Our paper of how we prepare and measure cryo-liquid jets has been published in the journal of Review of Scientific Instruments.
This article describes the features and the development of the experimental setup our collaborators from IOCB Prague (Jungwirth group), USC Los Angeles (Bradforth group), and the FHI (Winter group) have built to fit on our SOL³PES setup, as well as the obstacles that come with measuring photoelectron spectra from deeply cooled solutions like liquid ammonia. The paper was also selected as an Editorial Highlight (they call it Scilight). For more information, click here.
20.3.2020 BESSY II shutdown due to Corona virus pandemic
It was expected and on March 20 it became reality: BESSY II shut down completely due to the Corona virus. Our setup, which is currently attached at the beamline U49/2-PGM-1 is now in ‘hibernating mode’, hopefully not for too long…
1.2.2020 Welcome Wilson Quevedo!
Dr. Wilson Quevedo from the Diamond Light Source, United Kingdom, joint our team as postdoctoral fellow. A very warm welcome!
17.12.2019 Our paper on the electric double layer around aqueous TiO2 nanoparticles has been published in the ACS journal Applied Nano Materials
What a nice Christmas present. For the first time we could derive from spectroscopic measures the thickness of the TiO2 electric double layer (consisting of the Stern and the diffuse layer), which is about >0.8 nm. From a combination of XPS, partial fluorescence and partial electron yield measurements we could track the OH- spectroscopic fingerprints upon water dissociation and subsequently trapped around the TiO2 nanoparticles. It turned out that a significantly large amount of hydroxide is created, which is an indication for the high efficiency of water dissociation of the TiO2 catalyst, and on a wider perspective a promising result for H2 generation in true aqueous environments. For more details, click here.

The nightshift team (from left to right: Tillmann (now USC Los Angeles), Phil (ASCR IOCB Prague), Vojtech (ASCR IOCB Prague), Christian (FHI Berlin), Chin (UC Berkeley), Florian (DESY Hamburg/FHI Berlin), Pavel (ASCR IOCB Prague), Robert (HZB Berlin), and Bernd (FHI Berlin) successfully investigated liquid NaK alloys.
16.10.2019 Measuring liquid sodium-potassium alloys in a water atmosphere by PES
Once again, our collaborators from ASCR IOCB Prague, USC Los Angeles, UC Berkeley, and the Fritz-Haber-Institute Berlin, had a one-week beamtime at our SOL³PES station at BESSY II. This time they came up with the seemingly crazy idea to investigate (aqueous) solvated electrons created on top of a liquid sodium-potassium alloy inside a water atmosphere of roughly 1 mbar. Sodium-potassium alloys, or in short NaK, are highly reacting with water, forming among other species short-lived solvated electrons in high concentrations. Pavel and his team were especially interested in the phase transition between the blueish ‘solution-phase’ of single and di-solvated electrons and the golden ‘solution-phase’ of metallic solvated electrons. This has never been measured before with soft X-ray photoelectron spectroscopy. Well done!
17.9.2019 Dennis, Garlef und Martin at the E-MRS conference in Warsaw
Located at the beautiful capital of Poland, hosted by the University of Warsaw, this year’s European Materials Research Society gathered some 1.600+ participants for their fall meeting, including Dennis, Garlef, and Martin. Martin was selected to give a talk about his work on the operando investigation of the Solid-Liquid-Interface forming at the anode in lithium-ion batteries. His presentation was well received and triggered many stimulating questions.
Dennis and Garlef presented their newest results on the operando-based photoelectron spectroscopy investigations of iron-nickel oxyhydroxide catalysts for watersplitting during the poster session. There were a lot of scientists from the metal-oxide community present and both enjoyed the fruitful discussions. Overall the meeting was very well organized and scientifically very productive. On top the huge buffet was excellent.
4.9.2019 Dennis at the DPG summer school in Bad Honnef
Dennis joined a five-day summer school hosted by the German Physical Society at Bad Honnef in North Rhine-Westphalia, Germany. The summer school’s theme was energy science and the courses cover a broad range from energy demand, energy conversion, energy distribution and storage to social and environmental aspects – all crucial and highly relevant.
29.8.2019 Martin and Robert at the ACS meeting in San Diego
Martin and Robert joined the Fall meeting of the American Chemistry Society in sunny San Diego. It was Martin’s first visit to the United States. As there are roughly 80 sessions in parallel it is impossible to join all and therefore Martin and Robert split up and tried to attend as many talks as possible – Robert attended mostly (ambient-pressure) and operando-based XPS related talks, while Martin joined presentations covering newest (lithium-ion based) battery researches. It was an impressive and exhaustive, but worthwhile endeavor. A pitch-perfect event and programme, although a light jacket was needed in the sessions – due to freezingly 16 degrees Celsius temperature set by the convention center’s air conditioning.
29.7.2019 EE-NOGP at Tropical Islands
This year we went for our group excursion to Tropical Islands – Europe's largest tropical holiday resort just a 30 minutes’ drive South of Berlin. All members of EE-NOGP enjoyed the day, which wasn’t hard due to the many water attractions, water slides, mini golf, warm-water lagoons, and the longest white river in Europe.
19.7.2019 Dennis in clean room
Dennis got access to one of the clean rooms at HZB to deposit graphene on top of his holey Si3N4 membranes, which act as substrates for the electrocatalysts in contact with the electrolyte. The clean-room procedure should minimize contamination from small dust-particles, which might be present between the graphene and the membrane, leading eventually to fractures in the graphene and ultimately in a water leakage once transferred into vacuum. The yellow light in the lab is intentional, avoiding any short wavelengths in the spectrum that might cause photochemical reactions in photo-sensitive samples, which are present in the lab.
16.7.2019 Maintenance of the main turbopump from our SOL³PES setup
It was about time for another big service cycle for our main turbomolecular pump at the SOL³PES station. It is with mixed emotions seeing how unbelievably dirty this pump became during the last eight years since its previous major maintenance. During the BESSY shutdown Pfeiffer Vacuum will decontaminate the pump and make it shiny again, change its control unit and the bearing. We expect with the start of the new semester in October the SOL³ users will benefit from a renewed main turbo pump.
14.7.2019 Two-week flatjet beamtime at UE52-SGM
Bernds team from FHI was granted a two-week beamtime at the UE52-SGM-1 beamline with our SOL³ PES setup to measure systematically the photoelectron spectra and the photoelectron angular distribution from an aqueous flatjet. Two liquid microjets hitting under a distinct angle will produce a leaf-shaped flat water surface that is lacking the disadvantages of an cylindrical microjet. Bernds team got tons of new data, which looked very promising. More to come soon.
14.6.2019 The energy—recovering linac bERLinPro at BESSY is almost ready
Dennis, Garlef, and Robert got a tour through the ‘most sacred’ halls of the new energy-recovering linac (ERL), located in a new building next to the BESSY II storage ring. Once completed, the bERLinPro will demonstrate the feasibility of this highly advanced technology as a standard for future storage rings. More details of the bERLinPro project can be found here.
5.6.2019 Sebastian Beyer from the Chinese University of Hong Kong used our SOL³ setup to investigate ZIF-8 structure properties
Our collaborator Sebastian Beyer had a one-week beamtime to study spectroscopically the structural instabilities of a special class of zeolitic imidazolate frameworks, in short ZIF-8. To improve the acidic stability of ZIF-8, our collaborators from China, Australia, and the UK, incorporated functional groups on polypeptides. From a previous three-day beamtime with our SOL³ PES setup and from other complementary synchrotron investigations, we could demonstrate the enhanced acidic stability arises from the newly established coordinative interactions between the zink centers and the inserted carboxylate or phosphate groups – both groups have a lower pKa value than the imidazolate group. With enzymes, we also show a symbiotic stability reinforcement effect, i.e., the encapsulated biomolecules stabilize the ZIF matrix, while the ZIF exoskeleton protects the enzyme from denaturation. More details about this fascinating topic can be found in our article Improving the Acidic Stability of Zeolitic Imidazolate Frameworks by Biofunctional Molecules published in Chem.
8.4.2019 Dennis and Garlef at the Faraday Discussions in Ventura
Dennis and Garlef presented their work at the Ultrafast photoinduced energy and charge transfer: Faraday Discussions conference near the Marina of Ventura, California. It was for both the first-ever trip to the US. Overwhelmed by the impressions from California (and especially Los Angeles), it was also scientifically fruitful. A lot of new input from the time-resolved and ultrafast spectroscopy community for Dennis and Garlefs work on the (photo-)electrochemical investigations of metal-oxide catalysts for water splitting.
6.3.2019 Robert at MaxIV
Robert joined a one-week beamtime with the Prisle group from Oulu, Finland, at the brand-new synchrotron radiation facility MaxIV at Lund, Sweden. The state-of-the-art HIPPIE beamline offers near-ambient pressure photoelectron spectroscopy measurements from liquid solutions with high photon flux and a small focal spot. We were just the second ‘friendly users’ ever at this new beamline and it already worked flawlessly. Nønne Prisle and her team developed a model how (organic) molecules are distributed in the surface and interface of (water) droplets, which they wanted to verify at MaxIV. These data are very useful for refining our understanding of small water droplets in the atmosphere (cloud formation) and, on a bigger context, its influence on the climate change.
1.3.2019 Welcome Martin Schellenberger!
PhD candidate Martin Schellenberger from Sulzbach-Rosenberg, Bavaria, joined the team. A very warm welcome! Martin will perform soft X-ray transmission spectroscopy on silicon anodes for Li-ion batteries under operando conditions.
23.1.2019 Our photoelectron spectroscopy data of liquid ammonia has been published as a communication in JACS.
The first valence and core level spectra of liquid ammonia have been published in the Journal of the American Chemical Society. It was literally a tour-de-force to get the data measured. Together with our collaboration partners from Los Angeles (Steve, Ryan), from Prague (Pavel, Phil, Tillmann, Tomas, Krystof, Ondrej), and from the FHI in Berlin (Bernd, Christian, Heba, Claudia, Sebastian) we had a two-week beamtime at the UE52-SGM beamline in May 2018 and during the third night-shift we finally accomplished a stable microjet of liquid NH3. These results are an important step for the final goal of this project: the photoelectron measurements of solvated electrons in liquid ammonia in high concentrations. These measurements have been already performed and will be published soon.
14.1.2019 MatSEC meeting in Chorin
Once a year the PhD students in the graduate school MatSEC and their supervisors meet for a two-day retreat near Berlin. The students have to present their projects to the others and the supervisors give more or less helpful suggestions and recommendations. This year we all met in lovely Chorin – roughly 60 kilometers Northeast of Berlin. This municipality is famous for its more than 700 years old abbey. Although the schedule was very tight, we used some time of our lunch break to have a short visit of this monastery.
20.12.2018 Our article about the nature of water interactions with anatase titanium dioxide nanoparticles has been published in the Journal of Materials Chemistry A
Together with our collaborators from FHI, we investigated by soft X-ray PES, which molecular species exist at the titanium dioxide nanoparticle / aqueous solution interface as a function of solution pH. This solved a long-standing question whether water adsorbs dissociatively, molecularly, or mixed at the TiO2 interface. Our resonant photoemission results indicate that water adsorbs molecularly at low pH, and dissociatively at the TiO2 nanoparticle surface at basic pH. Water and OH- adsorb at the titanium sites, and no oxygen defects exist. For more details click here
29.11.2018 Solvated electrons in liquid ammonia measured
After a successful beamtime in May this year, where we recorded first-ever photoelectron spectra of liquid ammonia we went this beamtime one step further: together with our collaborators from Prague, FHI, and Los Angeles we dissolved lithium and sodium metal in liquid ammonia and studied the solvated electrons generated during this process. Unlike in water solvated electrons in liquid ammonia are stable for hours and the concentrations can be in the molar regime. Pavel, Phil, and Tillmann from Prague set up their customized liquid-ammonia preparation kit and liquid-jet system onto our SOL³ experimental station. After some troublesome first attempts, during the second night shift, we finally recorded it – the ground state energy of the solvated electron! Well done everyone!
27.11.2018 Our paper on organosulfur surfactants at the aqueous solution – vapor interface has been published in The Journal of Physical Chemistry C
Together with our collaborators from UC Irvine, California, and our ex-pat Stephan Thürmer in Japan we published a paper about the spatial arrangement of dimethyl sulfoxide, dimethyl sulfone, and dimethyl sulfite in water. It is part of the Hans-Joachim Freund and Joachim Sauer Festschrift in The Journal of Physical Chemistry C. Here, we present for the first time photoelectron angular distribution (PAD) measurements of solutes in water (so far we only published solvent PAD data) – these data are invaluable to understand how the DMSOx molecules coexist near the water surface in mixed equimolar aqueous solutions. The exceptionally large surface propensity of dimethyl sulfite is recognized by a narrow, gasphase-like photoelectron spectrum, revealing that dimethyl sulfite experiences very few hydration interactions. Experimentally observed trends in surface activity for the different molecules, which are complemented by molecular dynamics simulations, agree with findings obtained with other surface-sensitive techniques. For more details click here
1.11.2018 Our paper about how much ions do affect the electronic structure of liquid water has been published in Chemical Science
It is amazing – we are studying liquid water by more than 10 years now and still cannot answer so many of its mysteries. However, one question that bothered the community has been finally answered: What is the effect of solutes to the electronic-structure of liquid water? In other words: Do water’s electrons care about electrolytes? Together with our theory-collaborators from Prague (Eva, Štĕpán, and Petr), and the experimental groups from FHI (Heba, Marvin, and Bernd) and HZB (Iain) we investigated carefully how the binding energies of the liquid water peaks shift when increasing the concentration of sodium iodide from 0 to 8 molar. Surprisingly, the photoelectron spectrum is only mildly affected, with the largest (negative) shifts of ~370 meV for the 1b2 orbital and a narrowing of the flat-top shape orbital 3a1 of up to 450 meV. Most importantly, our calculations as well as our experiments show that due to electronic screening the 1b1 binding energy is only minimal shifted (by less than 60 meV) even at highest electrolyte concentrations, which makes this water peak a robust energetic reference for aqueous liquid microjet photoemission studies. For more details click here
16.10.2018 Lifting up our electron analyzer to replace a metal adapter
To solve some issues with our magnetic field compensation we replaced a 13.5 cm long adapter flange between the drift stage of our analyzer and the main chamber by a component of same length made of µ-metal. To get access, we had to lift up the analyzer with the BESSY crane and to remove parts of the Helmholtz-coils. The whole procedure went well and also thanks to updated lens tables, first measurements show an improvement in electron transmission, especially for lower kinetic energies.
1.9.2018 New Manipulators
Two new manipulators arrived and are ready to be used in the upcoming beamtimes for our users. The small one (a CF-40 with 5 cm translation) will replace our old liquid-jet manipulator, which served well the last eight (!) years. The big one comes with automated micrometer-adjustable xyz translation (up to 40 cm) and full rotation. It is meant for large sample(holders), transmission-cells, including (photo-)electrochemical cells with electron-transparent membranes and other bulky objects with up to 9.8 cm diameter that need to be investigated by photoelectron spectroscopy.
1.6.2018 Welcome Garlef Wartner!
PhD candidate Garlef Wartner from Stade, Lower Saxony, joined our team. A very warm welcome!
2.5.2018 First liquid ammonia spectra taken with our SOL³ setup
After many attempts at FHI and at BESSY we finally got a stable liquid ammonia jet running in our SOL³ setup under vacuum conditions. Phil and Tillmann managed to enhance the duration of the running jet to almost 8 hours – enough time to measure the valence band, core levels, angular photoelectron dependencies, salts dissolved in liquid ammonia at good statistics, and ultimatively, high-concentrated solvated electrons from dissolved Lithium metal in liquid ammonia. Please watch this video on Youtube and share the moment where we collected our first liquid ammonia photoelectron spectrum.
1.5.2018 Welcome Dennis Hein!
PhD candidate Dennis Hein from Aalen, Baden-Württemberg, joined our team. A very warm welcome!
30.4.2018 Our article about the water dissociation at the surface of hematite nanoparticles has been published in Chemical Science
Together with our colleagues from FHI we investigated the hematite nanoparticle – water interface by (resonant) liquidjet photoelectron spectroscopy. This method is sufficiently sensitive for the detection of adsorbed hydroxyl species, resulting from the water dissociation at the nanoparticle surface in aqueous solution. For more information click here
26.4.2018 First photoelectron spectra from a flat-jet
Bernd Winter and his team from FHI tested for the first time their new flat-jet assembly in our SOL³ setup under vacuum conditions. When two (identical) liquid-jets hit under a certain angle with the same flow a flat leaf-shaped liquid surface will form, which thickness can be adjusted to less than a micrometer. Bernds team also managed to get the first photoelectron spectra of liquid water from a flat-jet. Well done!
17.3.2018 Rotating the machine to magic angle
Over the weekend we needed to rotate our electron analyzer to 54.7° relative to the polarization axis of the synchrotron light at the U49/2-PGM-1 beamline of BESSY. At this so-called magic angle we can determine relative intensities from different solute species in aqueous solution from a liquid jet, which are then only depending on probing depth and ionization cross section, ignoring complex photoelectron angular distribution functions. We used this geometry of our electron analyzer to determine the depth-depending dissociation constant of HSO4-/SO42- in water as a function of concentration and kinetic energy. Rotating the machine only takes 10 minutes using a pulley assembly. All seven turbomolecular pumps of the HIPP-2 R4000 electron analyzer are off during the rotation, but the roughing pumps are still running, allowing for a fast run-up of the experiments compared to a complete venting.
14.3.2018 Dr. Stephan Thürmer from The University of Kyoto visited us
During his Europe-tour, Stephan Thürmer, very welcomed long-term expat and designer of the previous liquidjet experimental setup, the LiquidjetPES at BESSY, joined us for one day in our commissioning beamtime. As in the good-old times, Stephan got immediately familiar with our new SOL³ system and gave valuable input to improve the lens tables of our hemispherical electron analyzer, pushing the electron transmission to new highs.
13.12.2017 Dr. Alex Gaiduk from The University of Chicago visited us
Our theory collaborator from Giulia Gallis group at ‘UChi’, Alex Gaiduk, visited our group and took a look at our experimental setups at BESSY. He was impressed by the machines that confirm (and on rare occasions ‘readjust’) his invaluable calculated energies and redox potentials from aqueous salt solutions. He also presented his newest ab-initio calculations on one of the hot topics in the water-community: the precise energy position of liquid water’s conduction band - an important number that is experimentally very hard to derive. For more details on Alex work check out his newest article and the Galli group webpage.
13.11.2017 Our article about the iron-(III)-oxo oligomer formation in aqueous solution is online
Together with the research groups of Franziska Emmerling at the BAM Federal Institute for Materials Research and Testing, Berlin, Ralph Krähnert at the Technical University Berlin, and Bernd Winter at Fritz-Haber-Institut der Max-Planck-Gesellschaft, we looked into the formation process of small iron-oxo oligomers in aqueous solution. This chemical process is crucial for the formation of nanoparticles in aqueous phase. By a combination of Raman spectroscopy, Small Angle X-ray scattering and Liquid-microjet resonant photoelectron spectroscopy we could identify and measure the electronic structure properties of the iron-oxo oligomeric seeds that were produced by adding sodium hydroxide to our iron chloride solution. Our findings have been published in Physical Chemistry Chemical Physics. For more details click here.
28.9.2017 First gas dynamic virtual nozzle measurements with our setup
Together with our collaborators from FHI Berlin and IOM Leipzig we successfully measured for the first time at our setup a liquid jet, surrounded by a strong gas-stream to investigate gas-liquid interactions. Claudia, Bernd, and Sebastian from FHI were interested in the CO2 uptake of monoethanolamine in aqueous solutions and looked for its reaction products with respect to the gas-solution interface, and as a function of pH. An exciting experiment!
31.7.2017 Our new SOL³PES setup goes into user operation
Just in time for the start of the user operation of our new SOL³PES setup we got a technical article accepted in Review of Scientific Instruments, describing in detail its capabilities and features. Click here.
19.7.2017 Emmy-Noether meeting in Potsdam
Robert joined the annual three-day meeting as Emmy-Noether-‘freshman’, which took place for the 16th times. This year the current Emmy-Noether stipend holders and alumni met at the lovely Templiner See in Potsdam to talk about funding opportunities, career options after the Emmy-Noether stipend and the changes to the general social conditions in science and politics. For more details check out this DFG site (in German only).
10.4.2017 Beamtime at the U49/2-PGM-1 beamline at BESSY II
This beamtime we further explored metal oxide nanoparticles in water by liquidjet photoelectron spectroscopy. Arno prepared an Fe3O4 nanoparticle solution in our glove box – it is not an easy experiment. Jet stability and concentration are at their limits (and beyond…).
20.3.2017 Beamtime at UE52-SGM-1 beamline at BESSY II
We measured for the first time with our new SOL³PES setup angle-resolved valence photoelectron spectra of neat water. These measurements are key to derive the so-called anisotropy parameter of the outer water valence orbitals. By comparison with gas phase water anisotropy parameter values we can directly determine elastic and inelastic scattering behavior of the outgoing photoelectrons and indirectly the electron mean free path in water – a very important measure for the probing depth. To get our machine to the UE52-SGM-1 beamline we had to use the BESSY crane.
This beamtime was part of the 1st international photonschool at HZB, where 20 international postdocs, master and Phd students got hands-on experience on several experimental BESSY stations, including our SOL³ setup. See more
9.3.2017 Sensitive Method for Detecting Ion Pairs in Aqueous Solutions
Our work on Electron-Transfer Mediated Decay in aqueous solutions was published in Nature Chemistry. See more
1.3.2017 Welcome Arno Bergmann!
Dr. Arno Bergmann, from TU Berlin, joins the team as a postdoctoral research fellow. A very warm welcome!